
Is the runtime of a portable device directly related to the size of the battery and the energy it can hold? In most cases, the answer is yes. But with digital equipment, the length of time a battery can operate is not necessarily linear to the amount of energy stored in the battery.
In this article we examine why the specified runtime of a portable device cannot always be achieved, especially after the battery has aged. We address the four renegades that are affecting the performance of the battery. They are: declining capacity, increasing internal resistance, elevated self-discharge, and premature voltage cut-off on discharge.
Declining capacity
The amount of charge a battery can hold gradually decreases due to usage, ageing and, with some chemistries, lack of maintenance. Specified to deliver about 100% capacity when new, the battery eventually requires replacement when the capacity drops to the 70 or 60% level. The threshold by which a battery can be returned under warranty is typically 80%.
The energy storage of a battery can be divided into three imaginary sections consisting of available energy, the empty zone that can be refilled and the rock content that has become unusable. Figure 1 illustrates these three sections of a battery.

In nickel-based batteries, the rock content may be in the form of crystalline formation, also known as memory. Deep cycling can often restore the capacity to full service. Also known as 'exercise', a typical cycle consists of one or several discharges to 1 V/cell with subsequent discharges. This service is best performed with a battery analyser.
The loss of charge acceptance for the LiIon/polymer batteries is due to cell oxidation, which occurs naturally during use and as part of ageing. LiIon batteries cannot be restored with cycling or any other external means. The capacity loss is permanent because the metals used in the cells are designated to run for a specific time only and are being consumed during their service life.
Performance degradation of the lead acid battery is often caused by sulphation, a thin layer that forms on the negative cell plates, which inhibits current flow. In addition, there is grid corrosion that sets in on the positive plate. With sealed lead acid batteries, the issue of water permeation, or loss of electrolyte, also comes into play. Sulphation can be reversed to a certain point with cycling and/or topping charge but corrosion and permeation are permanent. Adding water to a sealed lead acid battery may help to restore operation but the long-term results are unpredictable.
Increasing internal resistance
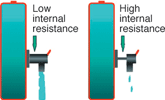
To a large extent, the internal resistance, also known as impedance, determines the performance and runtime of a battery. High internal resistance curtails the flow of energy from the battery to the equipment.
A battery with simulated low and high internal resistance is illustrated in Figure 2. While a battery with low internal resistance can deliver high current on demand, a battery with high resistance collapses with heavy current. Although the battery may hold sufficient capacity, the voltage drops to the cut-off line and the 'low battery' indicator is triggered. The equipment stops functioning and the remaining energy is undelivered.
NiCd has the lowest internal resistance of all commercial battery systems, even after delivering 1000 cycles. In comparison, NiMH starts with a slightly higher resistance and the readings increase rapidly after 300 to 400 cycles.
Maintaining a battery at low internal resistance is important, especially with digital devices that require high surge current. Lack of maintenance on nickel-based batteries can increase the internal resistance. Readings of more than twice the normal resistance have been observed on neglected NiCd batteries. After applying a recondition cycle with the Cadex 7000 Series battery analyser, the readings on the batteries returned to normal. Reconditioning clears the cell plates of unwanted crystalline formations, which restores proper current flow.
LiIon offers internal resistance characteristics that are between those of NiMH and NiCd. Usage does not contribute much to the increase in resistance, but ageing does. The typical life span of a LiIon battery is two to three years, whether it is used or not. Cool storage and keeping the battery in a partially charged state retard the ageing process.
The internal resistance of the LiIon batteries cannot be improved with cycling. The cell oxidation, which causes high resistance, is non-reversible. The ultimate cause of failure is high internal resistance. Energy may still be present in the battery, but it can no longer be delivered due to poor conductivity.
With effort and patience, lead acid batteries can sometimes be improved by cycling or applying a topping and/or equalising charge. This reduces the current-inhibiting sulphation layer but does not reverse grid corrosion.
Figure 3 compares the voltage signature and corresponding runtime of a battery with low, medium and high internal resistance when connected to a digital load. Similar to a soft ball that easily deforms when squeezed, the voltage of a battery with high internal resistance modulates the supply voltage and leaves the imprint of the load. The current pulses push the voltage towards the end-of-discharge line, resulting in a premature cut-off.
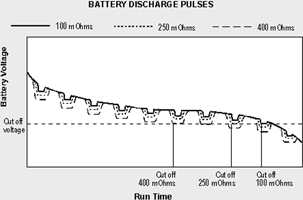
When measuring the battery with a voltmeter after the equipment has cut off and the load is removed, the terminal voltage commonly recovers and the voltage reading appears normal. This is especially true of nickel-based batteries. Measuring the open terminal voltage is an unreliable method to establish the state-of-charge (SoC) of the battery.
A battery with high impedance may perform well if loaded with a low DC current such as a flashlight, portable CD player or wall clock. With such a gentle load, virtually all of the stored energy can be retrieved and the deficiency of high impedance is masked.
The internal resistance of a battery can be measured with dedicated impedance meters. Several methods are available, of which the most common are applying DC loads and AC signals. The AC method may be done with different frequencies. Depending on the level of capacity loss, each technique provides slightly different readings. On a good battery, the measurements are reasonably close; on a weak battery, the readings between the methods may disperse more drastically.
Modern battery analysers offer internal resistance measurements as a battery quick-test. Such tests can identify batteries that would fail due to high internal resistance, even though the capacity may still be acceptable. Internal battery resistance measurements are available in the Cadex 7000 Series battery analysers.
Elevated self-discharge
All batteries exhibit a certain amount of self-discharge; the highest is visible on nickel-based batteries. These batteries discharge 10 to 15% of their capacity in the first 24 hours after charge, followed by 10 to 15% every month thereafter.
The self-discharge on the LiIon battery is lower compared to the nickel-based systems. The LiIon self-discharges about 5% in the first 24 hours and 1 to 2% thereafter. Adding the protection circuit increases the self-discharge to 10% per month.
One of the best batteries in terms of self-discharge is the lead acid system; it only self-discharges 5% per month. It should be noted, however, that the lead acid family also has the lowest energy density among current battery systems. This makes the system unsuitable for handheld applications.
At higher temperatures, the self-discharge on all battery chemistries increases. Typically, the rate doubles with every 10°C. Large energy losses occur through self-discharge if a battery is left in a hot vehicle. On some older batteries, stored energy may get lost during the course of the day through self-discharge rather than actual use.
The self-discharge of a battery increases with age and usage. For example, an NiMH battery is good for 300 to 400 cycles, whereas a NiCd adequately performs over 1000 cycles before high self-discharge affects the performance of the battery. Once a battery exhibits high self-discharge, little can be done to reverse the effect. Factors that accelerate self-discharge on nickel-based batteries are damaged separators (induced by excess crystalline formation, allowing the packs to cook while charging), and high cycle count, which promotes swelling in the cell.
At present, no simple quick-test is available to measure the self-discharge of a battery. A battery analyser can be used by first reading the initial capacity after full charge, then measuring the capacity again after a rest period of 12 hours. The Cadex 7000 Series performs this task automatically. In the future, quick test methods may be available that are able to measure the self-discharge of a battery within seconds.
Premature voltage cut-off
Some portable equipment do not fully utilise the low-end voltage spectrum of a battery. The equipment cuts off before the designated end-of-discharge voltage is reached and some precious battery power remains unused.
A high cut-off voltage problem is more widespread than is commonly assumed. For example, a certain brand of mobile phone that is powered with a single-cell LiIon battery cuts off at 3,3 V. The LiIon can be discharged to 3 V and lower. With a discharge to 3,3 V, only about 70% of the expected 100% capacity is utilised. Another mobile phone using NiMH and NiCd batteries cuts off at 5,7 V. The four-cell nickel-based batteries are designed to discharge to 5 V.

When discharging these batteries to their respective end-of-discharge threshold with a battery analyser after the equipment has cut off, up to 60% residual capacity readings can be retrieved. High residual capacity is prevalent with batteries that have elevated internal resistance and are operated at warm ambient temperatures. Digital devices that load the battery with current bursts are more receptive to premature voltage cut-off than analog equipment.
A 'high cut-off voltage' is mostly equipment-related. In some cases a battery with low voltage induces the problem of premature cut-off. A low table voltage is often caused by a battery pack that contains a cell with an electrical short. Memory also causes a decrease in voltage; however, this is only present in nickel-based systems. In addition, elevated temperature lowers the voltage level on all battery systems. Voltage reduction due to high temperatures is temporary and normalises once the battery cools down.
Summary
When observing the battery, there is no black and white, but many shades of grey. In fact, the battery behaves much like a human being. It is mystical, unexplainable and can never be fully understood. For some users, the battery causes no problems at all, for others it is nothing but a problem. Perhaps a comparison can be made with the aspirin. For some, it works to remedy a headache, for others the headache gets worse. And no one knows exactly why.
Increasingly, battery analysers are used to penetrate the mystery of the battery. Modern analysers, such as the Cadex 7000 Series, are capable of simulating the various digital load signatures and end-of-discharge thresholds voltages of wireless devices, medical instruments, laptops, video cameras and other portable devices. Using battery analysers provides a better understanding of this marvellous power source, the battery.
| Tel: | +27 11 466 1156 |
| Email: | [email protected] |
| www: | www.uniross.co.za |
| Articles: | More information and articles about Uniross Batteries |

© Technews Publishing (Pty) Ltd | All Rights Reserved